
Choosing the Right Materials for Transdermal Patches: A Step-by-Step Guide
Transdermal patches may look simple, but developing a safe, effective, and scalable product requires precise decisions about both materials and manufacturability.
When done right, they offer consistent dosing, non-invasive application, and a simpler way to improve medication adherence.

“The patient knows it’s applied, the caregiver knows it’s applied, and you avoid the issues that come with oral dosing,” says Gene McNamara, U.S. account manager and transdermal patch expert at Solventum™ (formerly part of 3M).
With McNamara’s help, we’ll explore step by step how to choose the right materials—a process shaped by drug compatibility, production realities, and regulatory demands. But first, let’s start with the basics.
What is a transdermal patch?
A transdermal patch is a drug delivery device applied to the skin that can deliver a controlled dose of medication into the bloodstream over time. The drug may be blended into the adhesive or held in a separate reservoir, depending on the patch design. These systems can offer a non-invasive alternative to oral medications or injections.
Why transdermal drug delivery is an important therapy
For the right medications, transdermal patches can offer a range of clinical and practical benefits like the following, making them an increasingly attractive option:
- Deliver medication non-invasively and painlessly
- Bypass the gastrointestinal tract, avoiding first-pass metabolism, the process by which a drug is broken down by the liver before reaching systemic circulation
- Enable more consistent dosing and may allow lower active ingredient levels
- Provide visible confirmation that a dose has been administered, helpful for both patients and caregivers
- Offer a safe alternative for patients with dysphagia, a condition that makes swallowing pills difficult and is more common in elderly populations
“A caregiver can see that the patch has been applied, and even mark the date or initials on the backing,” McNamara explains. “That’s especially valuable for patients with memory issues or diseases such as Alzheimer’s—and more generally, for elderly patients who may not remember to take oral doses.”
What’s inside a transdermal patch?
Now that we’ve covered what transdermal patches are and why they’re used, let’s look at how they’re built. While basic in appearance, each patch is a multi-layer system engineered for precision and reliability.
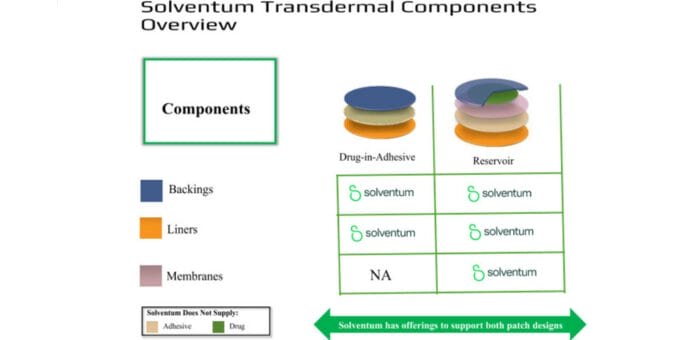
Common layers include:
- Backing. Provides structural support and protects the drug formulation.
- Adhesive. Attaches to the skin; may also carry the drug.
- Membrane. In some systems, controls the rate of drug release.
- Release liner. Protects the adhesive during storage and is removed just before application.
We’ll take a closer look at the two most commonly used types, drug-in-adhesive and reservoir, below.
The 5 steps to choosing the right transdermal materials
With that foundation in place, let’s walk through five key steps to selecting the right materials and designing a patch that performs successfully—from formulation to manufacturing.
Step 1: Match patch design to appropriate drug properties
Not all drugs are suitable for transdermal delivery. Ideal candidates tend to have:
- Low molecular weight. Typically under 500 Daltons.
- Lipophilicity. The ability to dissolve in oils, improving skin penetration.
- Low required dose. Patch size and skin absorption surface area are limited.
- Stability. The formulation must remain effective during production and over shelf life.
McNamara says the most successful transdermal drugs are oil-based, which improves compatibility with skin lipids. On the other hand, with certain materials, McNamara says this feature can also introduce potential issues, such as absorption into the patch backing.
Step 2: DIA vs. reservoir: Which patch design is right for your drug?
While other designs exist, the two most commonly used formats today are drug-in-adhesive (DIA) and reservoir.
DIA
In a DIA design, the active ingredient is blended directly into the adhesive layer and applied to a backing.
Benefits:
- Simplified construction and fewer layers
- Easier to manufacture
- Reduces overdose risk since there’s no membrane to fail
Challenges:
- Requires complex formulation to balance drug release, adhesion, and chemical stability
- Drug loading limited by adhesive properties
“DIA is more complicated to formulate,” says McNamara, “but pharma companies have shifted to DIA to reduce issues associated with membranes, which can become disrupted.”
Reservoir
In a reservoir patch, the drug formulation is housed between a backing and a rate-controlling membrane, which governs the release of medication through the skin over time. While these systems were foundational in early transdermal products, they still remain important for specific drug delivery challenges, particularly when extended release or solubility issues are a concern.
Benefits:
- Enables extended, controlled release
- Useful for actives with limited solubility
Challenges:
- More complex manufacturing
- Potential for dose spike with compromised seal
“Reservoir patches are still used in select applications,” McNamara explains. “They’re an important option for certain actives, and we continue to support them with our EVA-based rate-controlling membranes.” (See more on EVA below.)

Step 3: Select materials that won’t undermine drug delivery
Choosing the right materials, specifically backings, membranes, and liners, requires balancing drug compatibility, manufacturability, and long-term performance.
Backing materials
The backing layer is more than just structural support; it protects the drug from the external environment and gives the patch its overall look and feel. Its composition directly affects chemical compatibility, durability, and patient comfort.
Certain polymers like polyethylene (PE) or ethylene-vinyl acetate (EVA) may absorb oil-based drug formulations, reducing bioavailability over time. To help prevent this, McNamara says that Solventum uses multilayer laminate constructions that provide both function and form.
“Our laminate backings combine PET with a matte outer layer,” he explains. “That reduces absorption, minimizes reflection, and gives patients a discreet, low-profile patch.”
Backings must also withstand manufacturing forces—like heat, cutting, and tensioning—without distorting. That’s especially important for high-speed converting lines or multi-lane configurations where alignment is critical.
Membranes
In reservoir-style patches (see above), the membrane is the gatekeeper. It regulates how fast the drug moves from the reservoir to the skin. While membranes aren’t used in drug-in-adhesive systems, they remain essential in extended-release designs or when precise dosing is required for difficult actives.
Solventum’s membranes are made from non-porous EVA, and their performance is fine-tunable based on the vinyl acetate content and thickness. These attributes determine how the oil-based drug diffuses through the membrane over time.
“It’s a controlled soak-through, not passive micro-pores,” says McNamara. “The formulation should move consistently through the membrane, even under varying conditions.”
Material consistency, stability, and compatibility with both the drug and the reservoir material are all essential factors in choosing the right membrane for a given formulation.
Release liners
Release liners may seem simple, but they’re deceptively complex. The liner must adhere just enough to protect the adhesive during storage, but still peel away cleanly when it’s time to apply the patch.
McNamara says there are only a small number of chemistries that actually work to do that. “That’s true not only at the very beginning of making the patch but also in, say, two years, when it’s been sitting on the shelf and you still need to be able to remove the release liner.”
Adding to the challenge, liner materials must not interact with the drug formulation or leave behind residues that affect dosing.
Step 4: Designing for manufacturability from the start
A patch that works in the lab can fail at full scale, especially when working with thin, stretchy monolayer films.
“We consistently recommend Delta ModTech to customers. Their equipment is top notch, and they’ve worked extensively with our materials across a wide range of applications.”
Gene McNamara, U.S. Account Manager, Solventum
“These materials require advanced tension control and deep converting expertise,” says McNamara. “We consistently recommend Delta ModTech to customers. Their equipment is top notch, and they’ve worked extensively with our materials across a wide range of applications.”
Delta ModTech’s modular converting systems provide the servo-driven control, precision winding, and flexible integration needed to scale from prototype to production—while protecting sensitive materials.
Step 5: DMFs, PFAS, and the regulatory risks you can’t ignore
In the final stage of material selection, it’s not just about performance; it’s about meeting regulatory expectations that are evolving fast. Three of the most pressing concerns? DMFs, PFAS, and extractables.
Drug master files (DMFs)
A DMF allows a supplier like Solventum to confidentially submit detailed material data to the FDA. Drug manufacturers can reference the DMF in their filings without needing direct access to proprietary information.
“Without a DMF, most pharmaceutical companies won’t even consider the material,” says McNamara.
PFAS phase-out
Per- and polyfluoroalkyl substances (PFAS)—used in fluorinated liners—are under increasing regulation from both the EPA and global regulators due to health and environmental concerns.
Solventum is taking steps to eliminate PFAS related liners by the end of 2025.
Extractables and Elemental Impurities
Extractables are chemical compounds that can leach from patch materials—like adhesives or liners—under heat or stress, potentially compromising drug safety or stability.
“That’s one of the biggest things we’re seeing now—more attention on extractables and elemental impurities,” McNamara notes.
Nutraceutical patches are surging (but don’t skip material standards)
Nutraceuticals, an umbrella term for ingredients like CBD, melatonin, vitamins, and herbal extracts, are becoming more popular in patch format. These products are typically regulated as dietary supplements, which means they don’t go through the same FDA approval process as pharmaceuticals.
“Even for nutraceuticals,” McNamara notes, “consumers associate medical-grade materials with safety and quality. That matters.”
Nutraceutical companies that use high-integrity materials and trusted manufacturing processes are better positioned to win customer trust, and better prepared for any future regulatory shifts.
Final advice: Partner early, think long-term, and expect change
Formulating a successful transdermal patch demands more than clever chemistry. It’s a systems challenge. From drug compatibility and material performance to manufacturing scalability and regulatory foresight, every layer matters.
With so much at stake, choosing the right materials—and the right partners—can make the difference between a promising concept that stalls, and a viable, scalable product that hits the market efficiently.
Whether you’re launching a pharmaceutical patch or a wellness product, there are three essential takeaways:
- Work with experienced partners. Solventum and Delta ModTech, for example, each have decades of experience in this space.
- Design with scale in mind. Choose materials and equipment that can perform consistently from prototype to production.
- Stay proactive on regulation. This is especially relevant for extractables and DMF requirements.
Ready to build a better patch?
Delta ModTech partners with trusted suppliers like Solventum to help you qualify materials, refine processes, and scale with precision. Whether you’re developing a new drug delivery system or exploring the nutraceutical space, Delta ModTech can help you convert big ideas into production-ready success.
LEARN HOW WE CAN HELP IMPROVE YOUR MANUFACTURING PROCESS
Published on Sep 29 2025
Categories: Delta ModTech Blog, Materials, Transdermal
Previous Post
COMPAMED

